Aptorum Group Announces Further Positive Data on SACT-1 Against Neuroblastoma and Other Potential Tumor Types
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200831005392/en/
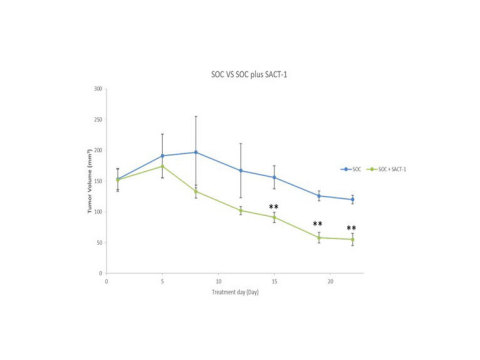
**Unpaired student’s t-test, p<0.01, n=8 (based on initial 22 days period) (Graphic: Business Wire)
Our repurposed drug candidate, SACT-1 is undergoing preparation for IND submission and is on track for regulatory application to target to commence phase 1b/2a clinical trials under the US FDA’s 505(b)(2) pathway.
“Neuroblastoma is one of the most prevailing solid tumor cancers in children, representing 8% - 10% of all childhood tumors, accounting for c. 15% of all cancer related deaths in the pediatric population1. For the high-risk patient group, the 5-year survival rate of this condition is around 40-50% as observed by the
Summary of our in vivo assessment against neuroblastoma and in vitro assessment against other cancers are discussed below.
Neuroblastoma In Vivo Assessment
Based on the initial 22 day data of a recent study we conducted in a xenograft mouse model of neuroblastoma, SACT-1 was orally administered daily at 60mg/kg in combination of SOC chemotherapy brought a statistically significant tumor shrinkage (unpaired student’s t-test, p<0.01) from Day 15 to Day 22, compared to the control group which received SOC only. Indeed, the combination reduced the tumor size by up to 54.2% in the first 22 days compared with the control (SOC only). SACT-1 appears to be effective in accelerating the effect of the SOC in early time points (from Day 1 - 7 vs control). This further supports our earlier in vitro observation that SACT-1 promotes tumor DNA damage and tumor cell death.
Other Cancer Types In Vitro Assessment
In addition, SACT-1 was also screened for in vitro activity in a panel of over 300 cancer cell lines. Similar to our previous findings against neuroblastoma cell lines, SACT-1 exhibits similar anti-tumor efficacy across one or more other major cancer types, including but not limited to colorectal cancer, leukemia and lymphoma cell lines. As a result, in addition to treating neuroblastoma, SACT-1 may have potential applications in the treatment of other cancers. Based on this discovery, the company plans to carry out further in vivo studies to study the efficacy of SACT-1 over other types of cancers to maximize the potential of SACT-1.
About SACT-1
As part of Aptorum Group’s SMART-ACT® platform, SACT-1 was discovered from our SMART-ACT® platform focused on orphan and unmet diseases. SACT-1 is a repurposed drug targeted for the treatment of neuroblastoma (and potentially other cancer types) especially in combination with SOC chemotherapy. SACT-1’s mechanism has been demonstrated in vitro to enhance DNA damage and tumor cell death.
About
For more information about
For further general presentation, please visit: https://ir.aptorumgroup.com/static-files/ca36cc65-6f23-4105-895e-f5f234ecca1e
Disclaimer and Forward-Looking Statements
This press release does not constitute an offer to sell or a solicitation of offers to buy any securities of
This press release includes statements concerning
As a result, the projections included in such forward-looking statements are subject to change and actual results may differ materially from those described herein.
This announcement is not a prospectus within the meaning of the Regulation (EU) n°2017/1129 of
This press release is provided “as is” without any representation or warranty of any kind.
2 https://www.cancer.org/cancer/neuroblastoma/detection-diagnosis-staging/survival-rates.html
View source version on businesswire.com: https://www.businesswire.com/news/home/20200831005392/en/
Investor relations
Investor Relations Department:
Tel: +44 020 80929299
Email: investor.relations@aptorumgroup.com
Redchip – Financial Communications United States
Investor relations
dave@redchip.com
+1 407 491 4498
Actifin – Financial Communications Europe
Investor relations
ggasparetto@actifin.fr
+33 1 56 88 11 22
Source: